When Stephen Plummer suggested to me that the HRS needed a Facebook page back in 2013 I was a little ambivalent because I am not really one for social media. Those who know me can attest to me also being a total technophobe - I don't 'do' mobile phones and struggle to understand Android technology on my Mother's iPad! Even so, I cautiously gave the 'go-ahead' if Stephen was happy to set the page up. It has been quite a revellation and now I am convinced that if run properly this sort of media definitely has a place in skills development and creation of a community with similar interests.
I liken the Facebook Group to a 'virtual society'. In some ways, these media have replaced the old Natural History Societies, although I think there remains a place for them because people do like to meet and I expect there would be demand for 'field meetings'. I did think of running such meetings but hit the immediate problem of insurance - these days you need insurance to run meetings on many sites (we get asked for evidence of insurance for DF meetings). So, we still need organisations such as Dipterists Forum - and we need people to join them and to participate in running them. I would urge members of the Facebook group to join DF and get involved in its events, or perhaps better still start to lead events locally for the FB group under the aegis of Dipterists Forum.
I follow several other Facebook groups and don't get the feeling that they have quite the same 'community' feel about them. I'm not wholly sure why this is but think it may be because they have a much wider focus. I also wonder whether the key to creating coherence is to provide feedback to the group about the information they are gathering. In the UKH and UKH larvae groups we do see a narrative developing. Other groups possibly need something similar.
So, here's to the UK Hoverflies Facebook Group (and many thanks Stephen). There is a great core of active members who I think will be able to keep the ethos going long after I have bowed out. Meanwhile, I think it is time to plan so activities that people can participate in. We had our Carrot Flower Challenge last year - I hope we have another attempt on this in 2018 with more rewarding results. We might get people in northern and western areas looking for Microdon larvae and of course it is possible that some people will head off to look for target species such as Caliprobola speciosa and Doros profuges.
This blog is intended as an occasional diary of information to feed back to hoverfly recorders in the UK and elsewhere. Inevitably there will be issues of interest that are in some way relevant to invertebrate ecologists and consequently I intend to use the medium as an opportunity to develop thoughts on pertinent topics.
Monday 25 December 2017
Sunday 24 December 2017
Time to develop a succession plan for the HRS
It is now 26 years since Stuart and I took on the role of scheme organisers for the Hoverfly Recording Scheme. We think we have made a reasonable difference and that we have given the scheme the necessary impetus to be forward-thinking and inclusive. Whilst our publications track record is a bit stuttering, we have managed to produce two provisional atlases and a species status review. We might have produced more papers, but that will hopefully be redressed in the coming months.
We are, however, only the custodians of the scheme. It is not our scheme and having made such a huge commitment we would like the scheme to live on well after us. If that is to happen we must recognise that there is a time to hand over the baton. That time is fast approaching. My health has taken a turn for the worse and both of us are itching to do things that will absorb a lot of our time and energy. We really need to start to hand over the reigns and to help to guide the scheme into a new management team. Our vision at this stage is that we should be booted up into the senior management - to be called upon for advice when needed, but not to do the day-to-day organisation and management. In other words we should become 'non-executive Directors' whilst a new team takes over the role of scheme organisers and figureheads.
We think there will need to be a transitional stage where we gradually ease out of the management chairs and let others take over; more than two people will be needed. If one looks at what is involved, there are several distinct strands:
The big question is how such a team should be organised? Do we need a formal or informal 'society'? Whilst we don't want to create a bureaucratic edifice, there is probably a need to develop a system that allows turnover of participants and recruitment of figureheads. As yet I don't have the answers, but I can pose lots of questions. The one thing I am clear about is that I cannot carry on in the role I provide - if I do so we will not find new 'leadership' and should I be unavailable the scheme would be rudderless; that would not be a good thing for one of the most dynamic and active schemes in the country.
We are, however, only the custodians of the scheme. It is not our scheme and having made such a huge commitment we would like the scheme to live on well after us. If that is to happen we must recognise that there is a time to hand over the baton. That time is fast approaching. My health has taken a turn for the worse and both of us are itching to do things that will absorb a lot of our time and energy. We really need to start to hand over the reigns and to help to guide the scheme into a new management team. Our vision at this stage is that we should be booted up into the senior management - to be called upon for advice when needed, but not to do the day-to-day organisation and management. In other words we should become 'non-executive Directors' whilst a new team takes over the role of scheme organisers and figureheads.
We think there will need to be a transitional stage where we gradually ease out of the management chairs and let others take over; more than two people will be needed. If one looks at what is involved, there are several distinct strands:
- Providing taxonomic advice and a source of specimen identification
- Validating and digitising data
- Managing incoming datasets and cleaning them before they are absorbed into the database
- Managing the database
- Writing newsletters
- Setting the sense of direction
- Identifying issues worthy of investigation and then undertaking the analysis
- Developing the website
The big question is how such a team should be organised? Do we need a formal or informal 'society'? Whilst we don't want to create a bureaucratic edifice, there is probably a need to develop a system that allows turnover of participants and recruitment of figureheads. As yet I don't have the answers, but I can pose lots of questions. The one thing I am clear about is that I cannot carry on in the role I provide - if I do so we will not find new 'leadership' and should I be unavailable the scheme would be rudderless; that would not be a good thing for one of the most dynamic and active schemes in the country.
Saturday 23 December 2017
Progress: British Flies - an introduction and key to families
In late November I posted a piece that was intended primarily as market research, but also to alert the world of Natural History to the possibility of a new guide to flies. There were lots of positive comments on my associated Facebook posts but also a few question marks as to whether such a book was really needed. Stuart and I firmly believe there is a need and that the moment is right to write such a book. Leave it any later and we may be too old (or gone). Even though there are quite a few Dipteists who could write such a book, most have other priorities. There are far fewer who have done as much testing of keys and understand the needs of novices. So, unless we do something nobody will!
Last Monday, Stuart and I met the publications unit of the Field Studies Council to discuss their possible role as publisher and to explore what they would want from us. I could not say anything in November about this meeting for obvious reasons. Our discussions were very positive and ended with an agreement that we (Stuart and I) would aim to deliver the package of text and illustrations by the end of 2019 or early 2020, witha view to publication in 2020. The timing is tight but we can do it provided we get cracking.
The really great thing about working with the FSC is that they understand keys and what works. They are also set-up to produce books that will have a long life-time in print and are not vastly expensive. The critical issue is to cover costs but there is not the same need for a particular profit margin as would be the case with a traditional publisher. The FSC model is to print sufficient books to meet expected market needs in the relatively short-term in order to minimise storage of large volumes of paper. The print run would be geared to anticipated demand and would be repeated at intervals when the need arose; thus keeping the book in print for as long as it was needed but allowing break points for revision if needed. We think the initial print run would be somewhere between 1,000 and 2,000 units. Printing would be in the UK, which has a positive impact on the book's carbon footprint and keeps jobs in the UK too. The basic proportions are as described previously:
- It will be a B4 book running to between 400 and 450 pages and composed of 3 sections:
- A substantial introduction. This would cover areas needed to understand the keys such as explaining the taxonomy and anatomical terms. An account of their life styles, biology and ecology of both adults and larvae, including treatment of medical and economic importance. Some very general coverage of the flies of macro-habitats.
- Keys to the families that occur in Britain. These have been rigorously tested and developed over the past 10 years. This key has been used in previous FSC courses run by us, as well a substantial number of other courses run by Dipterists Forum. We have some ideas about how to arrange the keys in ways that would make them a little less daunting to the novice.
- Descriptions of each family intended to be laid out in double page spreads with text on the left-hand page facing illustrations. The detail in each family description will vary, with some of the more obscure, small families perhaps having a half page with one illustration whilst the larger and better known families (especially those where there is a Recording Scheme) better covered with more text and several pages of photographs. Family descriptions will include coverage of the larval biology, where known, and a header summarising things like the number of species on the British list, their size range, the ease or difficulty of identification and whether it is covered by a Recording Scheme.
- It would NOT aim to cover all species, even in popular families, but should be regarded as a companion to such guides as the Larger Brachycera, Syrphidae and Tipuloideae.
- It would be illustrated by a combination of field photos, detailed photos of preserved specimens and line drawings to highlight specific features. It may not be possible to obtain field shots of some of the more obscure families especially where the species are very small. At this stage, we are anticipating full colour throughout, but we may have to re-think if printing costs are too high.
We discussed ideas such as crowd-funding and pre-publication offers. Happily there is no need for crowd-funding (at least we don't think so). There will be a pre-publication offer and we will be working to maximise the numbers of people who order a copy in advance - that should help to ensure that the book covers costs and that the shelves in Telford are not stacked high with unsold paper! It is too early to say much about price, but the market research from my last post indicated that the likely upper ceiling for such a volume would be in the £40-£50 range. We think that the final price should be somewhat below £40 and just possibly below £30 (subject to price changes in the next two years).
We have quite a bit of structural planning to complete and need to construct a project management chart to track progress - that is one of the jobs for me this Christmas. After that, we need to do some writing - the introduction is going to be a challenge. We have draft species accounts that need a thorough going-over by Graham and Tony. Nevertheless, I think we should have the text in place for peer-review by the end of 2018. The bigger challenge will be to populate the illustrations. I will be doing a lot of internet trawling this winter to establish what is available; after which we will just have to go and find the animals ourselves! That will be fun, but very demanding.
So, with any luck we will be providing a stocking-filler for Christmas 2020.
Friday 15 December 2017
Parataxonomy works!
Loyal readers (as with radio 4's 'More or Less') will recall that I have spent quite a bit of field work this year collecting fungus gnats and craneflies for their respective schemes. I have shown how I store the samples and have briefly reported back on the numbers of species recorded on my Scottish jaunt.
The other day, Peter Chandler sent me details of what I had recorded this autumn from my 'constant effort site' - Mitcham Common. It transpires that I did moderately well and recorded 120 gnat species and a further 11 species of Drosophilds. Not bad for a bit of parataxonomy.
So, here is a small chart to show how the list built up. In theory the list is heading towards a fairly low asymptote but I think this is really just a function of the time of year, with numbers rapidly diminishing in late October and November. There should be a new rise next year once the spring fauna kicks in, so it will be interesting to see what else I add next year. Peter tells me that the very richest sites have lists approaching 300 species, which is way beyond any of my expectations. Nevertheless, I am hopeful of getting maybe to 170 species.
The other day, Peter Chandler sent me details of what I had recorded this autumn from my 'constant effort site' - Mitcham Common. It transpires that I did moderately well and recorded 120 gnat species and a further 11 species of Drosophilds. Not bad for a bit of parataxonomy.
So, here is a small chart to show how the list built up. In theory the list is heading towards a fairly low asymptote but I think this is really just a function of the time of year, with numbers rapidly diminishing in late October and November. There should be a new rise next year once the spring fauna kicks in, so it will be interesting to see what else I add next year. Peter tells me that the very richest sites have lists approaching 300 species, which is way beyond any of my expectations. Nevertheless, I am hopeful of getting maybe to 170 species.
Wednesday 13 December 2017
What support do Recording Schemes need?
In a thread resulting from one of my earlier posts on the NFBR Facebook page, Steve Whitbread mentioned that the NFBR 2015 conference discussed what support might be given to Recording Schemes? I wonder how many schemes were there?
For me, the issue of external support is a difficult area. Many years ago, I was tasked with shifting the protected species role of English Nature in the Wakefield area to the voluntary sector. It did not go down at all well! Perhaps the biggest gripe was 'we used to do all of this until English Nature established a team of Protected Species Officers and insisted that it was their role and statutory duty' ... 'now you have no money it is conveniently being passed back to us'. I had a lot of sympathy but I was simply the messenger! We quickly found that it was not possible for the local Bat Groups to administer the volume of calls coming in and that we could not escape at least part of the workload!
I left the team about a year later and have no real idea how things panned out, but I really felt it was a bit much to expect volunteers to deal with the huge administrative burden of taking calls, organising a visits by somebody and then reporting back. That was not what the bat workers signed up to do - they loved bats and wanted to do something for them. I expect some dropped out as a result - and understandably so.
As a result of that experience and of dealing with the Humber Wildfowl Refuge - where I had to do the same thing, I realised that it was not a great idea for voluntary bodies to have too many ties to Government bodies, and especially logistical ties. That has made me quite fiercely independent and is one reason why the HRS tries to be as independent as possible - indeed we took on the scheme and did the initial data assembly because BRC did not have the resources to do the digitisation - so the problem goes a very long way back!
Having also been tasked with winding down support for Estuary Partnerships and been referred to as 'the man that wants to close Estuary Partnerships' the message is even starker - if you pump-prime a project that leads to people's jobs and livelihoods, you are setting yourself up for a fall when you cut funding. Somebody has to deal with that issue and I have had more than my fair share in my life! I would not wish it on anybody - it is not a career-maker it is the job given to somebody who will never be looked upon in a favourable light because all they bring to the Board papers is negative news on how nobody is picking up the reigns and taking over funding - it casts very unfavourably on you and undermines your morale!
So, when I hear talk of support for Recording Schemes I start to think about good and bad models. Any model that gives schemes the idea that they can just pass a job over to somebody else will lead to dependency and this will weaken rather than strengthen schemes. For the most part, it won't do much for schemes dealing with difficult taxa anyway because their biggest challenge is trying to cope with the vast volumes of data that ensue because the scheme is made accessible to people who don't have the taxonomic skills but do want to contribute. Taxonomic skills are the primary bottleneck in most of the invertebrate schemes I suspect. Synnergistic relationships with bigger schemes like butterflies, moths and dragonflies might be possible because there are probably enough skilled people to deflect some time into building relationships.
BUT, if you are the organiser of a scheme that barely anybody beyond a very small nucleus of specialists can ID, there is not much anybody can do to increase that nucleus without you actually having to take the job on! After all, they cannot do the training - it is you, the scheme organiser who will have to do it. And at a roughly 5% hit rate you will have to run an awful lot of courses to generate new recording skill, let alone people who are willing to take on some of the administrative burden.
So, my plea to anybody thinking about how to support schemes - please remember that in many ways, more support is actually likely to mean more work for the existing scheme organiser if it focusses on training and capacity-building! In this respect, the HRS does not need more support for capacity-building, it simply needs a few more people to take on the training role. If we had that capacity, there would also be a need to develop additional sets of specimens for use in courses (we have all the talks and a good method of teaching that we can teach to others). There might also be a need for another camera microscope and possibly access to a store of microscopes that could be couriered to the venue. So, it would certainly be worth looking into the logistics of setting up a pool of equipment that might be shared by a group of LERCS so that all the trainer had to do was to arrive with powerpoint presentations, course literature and relevant specimens (ours extend to 5 store boxes for hovers and 5 for general Diptera).
Printing course literature is useful further support - and here we must extend a HUGE thank you to BRC for prining the literature for our more recent Diptera courses (we have a charge for the hoverflies literature to cover replacement printing costs - not something we have called upon from BRC).
What else might be done? Well running a course a long way from home is VERY costly if you self-fund. When we went to Orkney we actually covered a sizeable part of the costs ourselves (DF covered part of it) - it could never have been done otherwise. Likewise when we went to Shetland where the LERC covered the ferry costs. Long-distance courses are expensive so a general fund to cover some of the costs might be helpful. BUT, after our experience in Orkney where we went believing that we had 8 participants, only to find that 3 had dropped out at the last minute, I would say all courses ought to involve an up-front charge to the participants.
Other thoughts include the need to provide capacity to develop teaching collections. Ours have been developed by me at my own expense - they comprise 10 store boxes now and probably 4-5,000 specimens. Just thinking about those costs - store boxes are now about £50 each. Staging pins about £0.5 each and micro-pins £0.2 each. At today's prices, our teaching collection would cost somewhere in the order of £850-£1000 not including the vast amount of my time compiling and maintaining the collection and of course a lot of my house storing it!
So, maybe NFBR should be thinking a lot more about what actually goes into training people, developing training for trainers and a fund for supporting courses and the assembly of necessary training material. This is a finite resource that, if it ceased, could be accommodated by the schemes - they just stop running courses - which means that the funding bodies see the direct impact of their cuts!
For me, the issue of external support is a difficult area. Many years ago, I was tasked with shifting the protected species role of English Nature in the Wakefield area to the voluntary sector. It did not go down at all well! Perhaps the biggest gripe was 'we used to do all of this until English Nature established a team of Protected Species Officers and insisted that it was their role and statutory duty' ... 'now you have no money it is conveniently being passed back to us'. I had a lot of sympathy but I was simply the messenger! We quickly found that it was not possible for the local Bat Groups to administer the volume of calls coming in and that we could not escape at least part of the workload!
I left the team about a year later and have no real idea how things panned out, but I really felt it was a bit much to expect volunteers to deal with the huge administrative burden of taking calls, organising a visits by somebody and then reporting back. That was not what the bat workers signed up to do - they loved bats and wanted to do something for them. I expect some dropped out as a result - and understandably so.
As a result of that experience and of dealing with the Humber Wildfowl Refuge - where I had to do the same thing, I realised that it was not a great idea for voluntary bodies to have too many ties to Government bodies, and especially logistical ties. That has made me quite fiercely independent and is one reason why the HRS tries to be as independent as possible - indeed we took on the scheme and did the initial data assembly because BRC did not have the resources to do the digitisation - so the problem goes a very long way back!
Having also been tasked with winding down support for Estuary Partnerships and been referred to as 'the man that wants to close Estuary Partnerships' the message is even starker - if you pump-prime a project that leads to people's jobs and livelihoods, you are setting yourself up for a fall when you cut funding. Somebody has to deal with that issue and I have had more than my fair share in my life! I would not wish it on anybody - it is not a career-maker it is the job given to somebody who will never be looked upon in a favourable light because all they bring to the Board papers is negative news on how nobody is picking up the reigns and taking over funding - it casts very unfavourably on you and undermines your morale!
The voice of experience!
So, when I hear talk of support for Recording Schemes I start to think about good and bad models. Any model that gives schemes the idea that they can just pass a job over to somebody else will lead to dependency and this will weaken rather than strengthen schemes. For the most part, it won't do much for schemes dealing with difficult taxa anyway because their biggest challenge is trying to cope with the vast volumes of data that ensue because the scheme is made accessible to people who don't have the taxonomic skills but do want to contribute. Taxonomic skills are the primary bottleneck in most of the invertebrate schemes I suspect. Synnergistic relationships with bigger schemes like butterflies, moths and dragonflies might be possible because there are probably enough skilled people to deflect some time into building relationships.
BUT, if you are the organiser of a scheme that barely anybody beyond a very small nucleus of specialists can ID, there is not much anybody can do to increase that nucleus without you actually having to take the job on! After all, they cannot do the training - it is you, the scheme organiser who will have to do it. And at a roughly 5% hit rate you will have to run an awful lot of courses to generate new recording skill, let alone people who are willing to take on some of the administrative burden.
So, my plea to anybody thinking about how to support schemes - please remember that in many ways, more support is actually likely to mean more work for the existing scheme organiser if it focusses on training and capacity-building! In this respect, the HRS does not need more support for capacity-building, it simply needs a few more people to take on the training role. If we had that capacity, there would also be a need to develop additional sets of specimens for use in courses (we have all the talks and a good method of teaching that we can teach to others). There might also be a need for another camera microscope and possibly access to a store of microscopes that could be couriered to the venue. So, it would certainly be worth looking into the logistics of setting up a pool of equipment that might be shared by a group of LERCS so that all the trainer had to do was to arrive with powerpoint presentations, course literature and relevant specimens (ours extend to 5 store boxes for hovers and 5 for general Diptera).
Printing course literature is useful further support - and here we must extend a HUGE thank you to BRC for prining the literature for our more recent Diptera courses (we have a charge for the hoverflies literature to cover replacement printing costs - not something we have called upon from BRC).
What else might be done? Well running a course a long way from home is VERY costly if you self-fund. When we went to Orkney we actually covered a sizeable part of the costs ourselves (DF covered part of it) - it could never have been done otherwise. Likewise when we went to Shetland where the LERC covered the ferry costs. Long-distance courses are expensive so a general fund to cover some of the costs might be helpful. BUT, after our experience in Orkney where we went believing that we had 8 participants, only to find that 3 had dropped out at the last minute, I would say all courses ought to involve an up-front charge to the participants.
Other thoughts include the need to provide capacity to develop teaching collections. Ours have been developed by me at my own expense - they comprise 10 store boxes now and probably 4-5,000 specimens. Just thinking about those costs - store boxes are now about £50 each. Staging pins about £0.5 each and micro-pins £0.2 each. At today's prices, our teaching collection would cost somewhere in the order of £850-£1000 not including the vast amount of my time compiling and maintaining the collection and of course a lot of my house storing it!
So, maybe NFBR should be thinking a lot more about what actually goes into training people, developing training for trainers and a fund for supporting courses and the assembly of necessary training material. This is a finite resource that, if it ceased, could be accommodated by the schemes - they just stop running courses - which means that the funding bodies see the direct impact of their cuts!
Tuesday 12 December 2017
Vice county recorders
Whenever the debate about local recording comes up, the usual answer is that there are VC recorders who should be the links between LERCS and local recorders. Or that the VC recorders can take on verification of records. It makes me wonder whether there is really any understanding of what goes on in many taxonomic groups? Yes, BSBI have VC recorders, so do Butterfly Conservation and perhaps also the National Moth Recording Scheme and possibly British Dragonfly Society. Some Societies such as the YNU and LNU have recorders but in many places there is no Naturalists Union - those societies died years ago in many parts of the country. I know at least one VC moth recorder who has extracted himself and there is no replacement.
So, to explain the situation for Diptera, which is probably pretty similar for BWARS. We have a small network of active hoverfly recorders - until 2011 50% of the data was supplied by just 20 people. Today the proportions are a little better and maybe about 50 people fulfil this role. BUT, for example, in 2017 I generated over 5,000 records - nearly 10% of the total data for the year! Hoverflies are the flagship family of Diptera and so if we have between 20 and 50 really active recorders you can bet it is down below 10 for anything beyond the most popular families!
So, with about 50 really active recorders that does not even equate to one per Vice-County! maybe one to every two VCs. But then there are several clumpings of activity - for example, four of the major contributors to the HRS live within a 30 mile radius of Peterborough, there were three in Dorset (two have effectively retired this year), two in Devon ... There is not an even spread. Of those, the majority have other interests in Diptera and although they are very productive they also contribute to many other Recording Schemes. So, the reality is that there is neither the spread of capacity, nor the numbers who are willing to get involved at a VC level. And as one very capable recorder said to me when I tried to get him to become part of the HRS team 'I like the fieldwork but don't want to become an administrator'.
So, as my offering for today, I think it is time that the shakers and movers in biological recording stopped assuming that there are networks of recorders for all taxa. Most schemes are pretty well 'one-man-bands' or at best a few enthusiasts with a small circle of semi-active contributors. For example, this year, apart from hoverflies, I have collected data for fungus gnats and craneflies - but only by collecting specimens for the Scheme Organiser to ID. Probably no more than five people in the UK are capable of doing fungus gnats with any competency and most of those will seek advice from the scheme organiser. Craneflies have a few more capable people but I would doubt more than 20 (if that). I also hold on to Pipunculids for David Gibbs, but not that many as they are a pain because the head falls off so easily and makes curation difficult.
There are other popular groups that do have a wider following - I do Sciomyzids, Scathophagids and Larger Brachycera as a matter of course plus a few (not more than a couple of hundred) Tachinids, Dolis and Empids and a scattering of Lauxaniids, Heleomyzids and Sepsids. There are a few other families that I'm building up collections to do in due course but they won't add up to much. And I am one of the more active recorders - most people don't do anything along my lines (I also collect a few beetles for schemes too and have just offered the weevil Scheme my assistance when on safari). Who is going to do the liaison for the several dozens of VCs that I have visited this year? I'll probably have to do it myself because there is nobody!
So, time to start to realise that recording beyond the popular taxa is not awash with VC recorders - it is a very thin spread of people who do the best they can and are finding it increasingly difficult to keep on top of the administrative workload. I know I never expected to find myself giving so many hours to running the HRS and had I known then what I know now I might have opted to keep quiet and do my own thing! Too late now tho'!
So, to explain the situation for Diptera, which is probably pretty similar for BWARS. We have a small network of active hoverfly recorders - until 2011 50% of the data was supplied by just 20 people. Today the proportions are a little better and maybe about 50 people fulfil this role. BUT, for example, in 2017 I generated over 5,000 records - nearly 10% of the total data for the year! Hoverflies are the flagship family of Diptera and so if we have between 20 and 50 really active recorders you can bet it is down below 10 for anything beyond the most popular families!
So, with about 50 really active recorders that does not even equate to one per Vice-County! maybe one to every two VCs. But then there are several clumpings of activity - for example, four of the major contributors to the HRS live within a 30 mile radius of Peterborough, there were three in Dorset (two have effectively retired this year), two in Devon ... There is not an even spread. Of those, the majority have other interests in Diptera and although they are very productive they also contribute to many other Recording Schemes. So, the reality is that there is neither the spread of capacity, nor the numbers who are willing to get involved at a VC level. And as one very capable recorder said to me when I tried to get him to become part of the HRS team 'I like the fieldwork but don't want to become an administrator'.
So, as my offering for today, I think it is time that the shakers and movers in biological recording stopped assuming that there are networks of recorders for all taxa. Most schemes are pretty well 'one-man-bands' or at best a few enthusiasts with a small circle of semi-active contributors. For example, this year, apart from hoverflies, I have collected data for fungus gnats and craneflies - but only by collecting specimens for the Scheme Organiser to ID. Probably no more than five people in the UK are capable of doing fungus gnats with any competency and most of those will seek advice from the scheme organiser. Craneflies have a few more capable people but I would doubt more than 20 (if that). I also hold on to Pipunculids for David Gibbs, but not that many as they are a pain because the head falls off so easily and makes curation difficult.
There are other popular groups that do have a wider following - I do Sciomyzids, Scathophagids and Larger Brachycera as a matter of course plus a few (not more than a couple of hundred) Tachinids, Dolis and Empids and a scattering of Lauxaniids, Heleomyzids and Sepsids. There are a few other families that I'm building up collections to do in due course but they won't add up to much. And I am one of the more active recorders - most people don't do anything along my lines (I also collect a few beetles for schemes too and have just offered the weevil Scheme my assistance when on safari). Who is going to do the liaison for the several dozens of VCs that I have visited this year? I'll probably have to do it myself because there is nobody!
So, time to start to realise that recording beyond the popular taxa is not awash with VC recorders - it is a very thin spread of people who do the best they can and are finding it increasingly difficult to keep on top of the administrative workload. I know I never expected to find myself giving so many hours to running the HRS and had I known then what I know now I might have opted to keep quiet and do my own thing! Too late now tho'!
Monday 11 December 2017
On the issue of data verification
My last couple of posts on data verification and LERCS have generated useful threads on the NFBR Facebook group. Writing these entries has been/is a way of generating some thinking and possibly teasing out issues that I hope will get people thinking and maybe get the shakers and movers in NFBR and LERCS to think in a somewhat broader manner.
I'm sure there are plenty of people who are not directly involved in the LERC/NBN network of professionals that are unaware of the relationship between the two, and between them and the recording schemes and vice-versa. That is probably especially true for the many specialist recorders who are mainly interested in their subject area and not in what happens to their records (apart from making them available in as convenient a manner as possible - to them).
I recall in the old ISR days that Pete Kirby once totted up all of the recording schemes that he would have to contact in order to submit his records on a yearly basis. It ran into several dozens (Pete is one of the World's great polymaths). The same holds if you spread your wings and record across many counties - having to split data up and submit directly to each LERC is also a chore that many don't want or find a disincentive to submit data. My own excuse for not submitting data in this way is that it is such a big job that I would rather place my data directly with the NBN - but of course it is unverified! Most of it will be OK but there will be glitches amongst families where I am less familiar.
So that brings me to the issue of how do you verify data at a gross scale? Unless you go through every single record and voucher specimen you can never be quite sure whether the data are trustworthy. And, even when you do, mistakes will occur. So, one needs a screening process based on whether somebody is known to the major specialists in the local area or nationally.
I would split records from a single individual into a hierarchy:
I would start with 'what do we know about this person?' If they are a well-known and respected specialist then the chances are that their records are reliable and can be entered without too much concern provided they don't stray too far from the areas where they have known expertise.
But, if they are an unknown entity then you perhaps need to check further before entering records. If you don't know them, do the shakers and movers in the groups they mainly covered know them and have a feel for their reliability? For example, I have on several occasions happened across people who I did not know but had heard of - in the field making snap identifications on species that cannot be done without microscopic examination. They produce lots of data and a LERC might think they were a real expert, but in reality a great deal of their data is junk!
One of the critical issues with data is that the entries made by the recorder might have been fine at the time they made their record, but the taxonomy has moved on - but were they aware of it? If they worked in isolation, were unknown to other specialists and never corresponded with others then I would raise a big question-mark? If their records were made before a major split and they died before that split occurred, you cannot be at all sure what they actually recorded. Some very simple early validation is therefore possible - are the data taxonomically up to date? If not, seek the advice of recording scheme organisers.
So, the next question must be - it is fine to have volunteers entering data - very necessary, but before those data are passed to the volunteer for entering (or paid staff member), somebody needs to evaluate the likely reliability of the data and the problems that might be encountered. The older the data, the more likely it is to have glitches, either because the taxonomy has moved on, or because the keys were more challenging and open to misinterpretation. As such, these data should be flagged as requiring verification BEFORE being made publicly available.
Thus, we hit the usual problem - there is a vast backlog of data to assimilate, and a very small number of people who can make a reliable judgment as to the veracity of the records, so there is a need to establish screening and prioritisation. Digitising data from a national figure must take priority over the scrappy notebooks of somebody who was unknown, worked alone and may or may not have produced reliable records. If in doubt, contact the national schemes to see what they know about somebody and whether they can point to potential problem areas.
I'm sure there are plenty of people who are not directly involved in the LERC/NBN network of professionals that are unaware of the relationship between the two, and between them and the recording schemes and vice-versa. That is probably especially true for the many specialist recorders who are mainly interested in their subject area and not in what happens to their records (apart from making them available in as convenient a manner as possible - to them).
I recall in the old ISR days that Pete Kirby once totted up all of the recording schemes that he would have to contact in order to submit his records on a yearly basis. It ran into several dozens (Pete is one of the World's great polymaths). The same holds if you spread your wings and record across many counties - having to split data up and submit directly to each LERC is also a chore that many don't want or find a disincentive to submit data. My own excuse for not submitting data in this way is that it is such a big job that I would rather place my data directly with the NBN - but of course it is unverified! Most of it will be OK but there will be glitches amongst families where I am less familiar.
So that brings me to the issue of how do you verify data at a gross scale? Unless you go through every single record and voucher specimen you can never be quite sure whether the data are trustworthy. And, even when you do, mistakes will occur. So, one needs a screening process based on whether somebody is known to the major specialists in the local area or nationally.
I would split records from a single individual into a hierarchy:
- Groups with which they are most familiar and therefore least likely to make mistakes
- Groups that they look at intermittently and are more likely to make mistakes
- Groups that they rarely look at and probably only note if something has caught their eye (e.g. something charismatic and unusual).
So, where can the LERCS start when they get a set of diaries from a recorder?
I would start with 'what do we know about this person?' If they are a well-known and respected specialist then the chances are that their records are reliable and can be entered without too much concern provided they don't stray too far from the areas where they have known expertise.
But, if they are an unknown entity then you perhaps need to check further before entering records. If you don't know them, do the shakers and movers in the groups they mainly covered know them and have a feel for their reliability? For example, I have on several occasions happened across people who I did not know but had heard of - in the field making snap identifications on species that cannot be done without microscopic examination. They produce lots of data and a LERC might think they were a real expert, but in reality a great deal of their data is junk!
One of the critical issues with data is that the entries made by the recorder might have been fine at the time they made their record, but the taxonomy has moved on - but were they aware of it? If they worked in isolation, were unknown to other specialists and never corresponded with others then I would raise a big question-mark? If their records were made before a major split and they died before that split occurred, you cannot be at all sure what they actually recorded. Some very simple early validation is therefore possible - are the data taxonomically up to date? If not, seek the advice of recording scheme organisers.
So, the next question must be - it is fine to have volunteers entering data - very necessary, but before those data are passed to the volunteer for entering (or paid staff member), somebody needs to evaluate the likely reliability of the data and the problems that might be encountered. The older the data, the more likely it is to have glitches, either because the taxonomy has moved on, or because the keys were more challenging and open to misinterpretation. As such, these data should be flagged as requiring verification BEFORE being made publicly available.
Thus, we hit the usual problem - there is a vast backlog of data to assimilate, and a very small number of people who can make a reliable judgment as to the veracity of the records, so there is a need to establish screening and prioritisation. Digitising data from a national figure must take priority over the scrappy notebooks of somebody who was unknown, worked alone and may or may not have produced reliable records. If in doubt, contact the national schemes to see what they know about somebody and whether they can point to potential problem areas.
Sunday 10 December 2017
In praise of the Local Environmental Records Centres
Yesterday's post elicited quite an interesting thread concerning LERCS and their role in biological recording. It reminds me of the debate than ensued after Natural England cut its funding for LERCS in favour of more centralised data management. I felt at the time that, whilst NE had some good points about the need for LERCS to put data onto the NBN, they had completely missed the point about what LERCS are about and the role they play in assembling data. I fear that this extends further because the NBN is the user interface for most biological recording and is at arms length from the actual contributors of data.
Those who read my posts will have realised a long while ago that my approach is more about data contributors than data users and yesterday's responses convince me more than ever that the focus is too heavily on how data can be accessed and used. That is understandable in many ways - the NBN would not have been funded had there not been a demand for data. BUT, once the infrastructure is in place it is necessary to think about how it is populated and supplied.
The NBN is, undoubtedly, an asset to all users including the recording community so let us not think that there are specific sectoral interests at work. We all need and use it. Those who use the NBN but not the LERCS do not see what goes into the regional element of biological recording. If you are a keen naturalist and not inclined towards a specific recording scheme where is your natural community? You can of course sit on your own and upload your data onto iRecord. I suspect that is not terribly rewarding because you may wait months or years to see your data verified, and perhaps even longer before it is put to good use. Also, do you interact with a human being, let alone somebody with whom you can share your passion? Simple answer NO - it is a machine and you are simply one of the suppliers of material with which to make sausages!
Getting involved with a LERC is a different matter - they are the focus for a community, provide a centre for all sorts of activities and help to create the social interaction that the majority of human beings need. We are a naturally social animal that is increasingly living in isolation, so LERCS are an important social asset. That is BEFORE they start to assemble data, run programmes and interact with planning authorities and developers.
Without the LERCS I wonder if there would be anything like the data that currently exist - probably not! I was reminded that there is a whole generation of recorders who are getting a bit old but still have extensive notebooks that need transcribing - so where a LERC facilitates this, there is a long-term and extensive social gain. Those that run courses provide another valuable conduit for recording schemes to grow skills across the country - the HRS has done this in many places and is heavily reliant upon the LERCS for this facilitation.
We should also stop and think - if there were no LERCS, would we, the Recording Scheme oragnisers, be bombarded by consultants wanting information? I guess there is a risk and jolly well hope that never happens. I for one have no desire to add that job to the role of scheme organiser! So, for me, the LERCS are an essential part of the infrastructure.
My big worry is not the LERCS but who they use to verify data. There seem to be sizeable Diptera datasets - but who is supplying the data - there are relatively few active Dipterists in Dipterists Forum and although I do know of a few who are not members, it begs the question that if you are an active Dipterist and don't engage with DF are you really making the best use of the communal skill to verify your own work? We all need an element of peer-review and learn a great deal from each other. Or, are the data being verified? Clearly some is not, and quality control is quite patchy. I'm not volunteering to do more - there is enough for me to do already!
But that brings us back to the perennial question - there is a lot of focus on the users of data and organisers of data but precious little appreciation of where the data come from and what makes recorders tick. The NBN is simply a vehicle for disseminating data, whilst LERCS are there in part to motivate the boots on the ground (as are Recording Schemes).
Those who read my posts will have realised a long while ago that my approach is more about data contributors than data users and yesterday's responses convince me more than ever that the focus is too heavily on how data can be accessed and used. That is understandable in many ways - the NBN would not have been funded had there not been a demand for data. BUT, once the infrastructure is in place it is necessary to think about how it is populated and supplied.
The NBN is, undoubtedly, an asset to all users including the recording community so let us not think that there are specific sectoral interests at work. We all need and use it. Those who use the NBN but not the LERCS do not see what goes into the regional element of biological recording. If you are a keen naturalist and not inclined towards a specific recording scheme where is your natural community? You can of course sit on your own and upload your data onto iRecord. I suspect that is not terribly rewarding because you may wait months or years to see your data verified, and perhaps even longer before it is put to good use. Also, do you interact with a human being, let alone somebody with whom you can share your passion? Simple answer NO - it is a machine and you are simply one of the suppliers of material with which to make sausages!
Getting involved with a LERC is a different matter - they are the focus for a community, provide a centre for all sorts of activities and help to create the social interaction that the majority of human beings need. We are a naturally social animal that is increasingly living in isolation, so LERCS are an important social asset. That is BEFORE they start to assemble data, run programmes and interact with planning authorities and developers.
Without the LERCS I wonder if there would be anything like the data that currently exist - probably not! I was reminded that there is a whole generation of recorders who are getting a bit old but still have extensive notebooks that need transcribing - so where a LERC facilitates this, there is a long-term and extensive social gain. Those that run courses provide another valuable conduit for recording schemes to grow skills across the country - the HRS has done this in many places and is heavily reliant upon the LERCS for this facilitation.
We should also stop and think - if there were no LERCS, would we, the Recording Scheme oragnisers, be bombarded by consultants wanting information? I guess there is a risk and jolly well hope that never happens. I for one have no desire to add that job to the role of scheme organiser! So, for me, the LERCS are an essential part of the infrastructure.
My big worry is not the LERCS but who they use to verify data. There seem to be sizeable Diptera datasets - but who is supplying the data - there are relatively few active Dipterists in Dipterists Forum and although I do know of a few who are not members, it begs the question that if you are an active Dipterist and don't engage with DF are you really making the best use of the communal skill to verify your own work? We all need an element of peer-review and learn a great deal from each other. Or, are the data being verified? Clearly some is not, and quality control is quite patchy. I'm not volunteering to do more - there is enough for me to do already!
But that brings us back to the perennial question - there is a lot of focus on the users of data and organisers of data but precious little appreciation of where the data come from and what makes recorders tick. The NBN is simply a vehicle for disseminating data, whilst LERCS are there in part to motivate the boots on the ground (as are Recording Schemes).
Saturday 9 December 2017
Should unverified data be placed on the NBN?
In previous posts I have drawn attention to the problem of 'dodgy' records on the NBN. It is an issue that vexes some and is of little concern to others, but I wonder whether it has really been properly thought out.
A recent thread on the NBN Facebook page allowed commentators to observe that there seemed to be greater emphasis on data users than on data providers by the NBN. Whether that is true or not I cannot be sure, but there is an issue if the data providers start to feel that way! I think that part of the problem is that there is a growing dichotomy between the 'professionals' in the biodiversity data industry and the technical specialists, most of whom do so on a voluntary basis.
So, lets start by asking a few questions:
What experience do you need to be a LERC data manager?
Well, I guess the highest priority must be an ability to manage data - i.e. understand the nuts and bolts of RECORDER 6 and be able to drive it to produce the reports that clients need. You probably need to be good with people - to get your local recording community to contribute records so that you have something to sell to commercial clients. By implication, it is clear that you cannot be a technical specialist across all taxa - the Eric Philps of the world are very few and far between, and are probably not ideal database managers!
So, what are you aiming for? As much data as possible - although you cannot say it, the commercial imperative is quantity and not quality. Also, you probably want to concentrate on the data that is most commercially useful - bats, badgers great-crested newts, Schedule 41/42 species, BAP species.
What experience do you need to run a recording scheme?
Probably the most important requirement is an innate interest in the group of organisms that you are proposing to record. If you are taking over an existing scheme then you probably also need to have the confidence of fellow recorders that you know what you are talking about, or are able (and willing) to seek the input of others more skilled than yourself. Data management skills are desirable but not a pre-requisite (although you will become unstuck in due course if you don't gain skills). Like the LERC manager, you also need to be capable of motivating people, but more importantly you must understand how important it is to give structured feedback to your contributors: schemes that act as a black hole rapidly lose impetus (and there are good many such schemes). As a scheme organiser you must also be confident enough to challenge incoming records, no matter who from, so you MUST develop the necessary taxonomic, ecological and biogeographic skills. If you run a national scheme it also helps to have a reasonable knowledge of the landscape and ecology of the whole of the British Isles (but that can come with time).
What are you intending to produce with the data?
Here I think the biggest divergence probably obtains between the LERC and the Recording Scheme.
Unless the LERC holds data and is seen as a suitable source of information it will not get the income to survive - so financial survival is critical. You will not necessarily be judged on data quality - to many clients the important thing is enough information to satisfy the local planners that you have adequately investigated the environmental parameters of your proposal. For the NBN, an ability to list the vast numbers of records at a broad taxonomic scale is probably pretty important to attract ongoing statutory agency funding. In other words, nobody is starting from the question of data quality - it is headlines that grab the politicians and it is the politicians that control the purse-strings. And, as the vast majority of politicians have no scientific knowledge whatsoever and could not interpret a graph if it hit them in the face, big means best!
For the Recording Scheme, the most obvious and stinging criticism is that the dataset contains anomalies. The second most damaging problem is a failure to get data organised and maps and other outputs transmitted to your supporters (in other words they have a different set of supporters). If the supporters lose faith in the scheme then they will not engage and the scheme will decline. If it is vibrant and producing lots of outputs and creating a community around the interest group, more records will be generated and reliable recorders will be recruited. So, a vibrant recording scheme has got to be there to create a community spirit and to provide motivational feedback.
How many LERC and NBN staff run recording schemes or contribute records out of office hours?
I doubt this one can be quantified. I do know that at least some are occasional contributors to schemes. But in a parallel situation, I was always amazed at the low level of interest in biological recording amongst colleagues at Natural England. True, there were plenty of twitchers but real recorders were at a premium. Precious few former colleagues sit amongst the major or even minor contributors to the HRS (except former CSD and EFU staff who make up a significant part of the key major recorders). It was always a disappointment to me to hear that people felt that they did not want to take their job home with them but were happy to sit in meetings talking about what the recording schemes could be tasked with.
Unless some attention is paid to data quality, users of the NBN will start to become wary of its content. If the data are unreliable, then so too are the outputs of the science that is based on those data. In the past, the HRS has flagged dodgy records with LERCs. Some have taken action, but others have ignored our advice - so we find it best not to access those datasets because we have to go through the same process time and again. We no longer bother to provide that feedback except where we know LERC managers will act.
A recent thread on the NBN Facebook page allowed commentators to observe that there seemed to be greater emphasis on data users than on data providers by the NBN. Whether that is true or not I cannot be sure, but there is an issue if the data providers start to feel that way! I think that part of the problem is that there is a growing dichotomy between the 'professionals' in the biodiversity data industry and the technical specialists, most of whom do so on a voluntary basis.
So, lets start by asking a few questions:
What experience do you need to be a LERC data manager?
Well, I guess the highest priority must be an ability to manage data - i.e. understand the nuts and bolts of RECORDER 6 and be able to drive it to produce the reports that clients need. You probably need to be good with people - to get your local recording community to contribute records so that you have something to sell to commercial clients. By implication, it is clear that you cannot be a technical specialist across all taxa - the Eric Philps of the world are very few and far between, and are probably not ideal database managers!
So, what are you aiming for? As much data as possible - although you cannot say it, the commercial imperative is quantity and not quality. Also, you probably want to concentrate on the data that is most commercially useful - bats, badgers great-crested newts, Schedule 41/42 species, BAP species.
What experience do you need to run a recording scheme?
Probably the most important requirement is an innate interest in the group of organisms that you are proposing to record. If you are taking over an existing scheme then you probably also need to have the confidence of fellow recorders that you know what you are talking about, or are able (and willing) to seek the input of others more skilled than yourself. Data management skills are desirable but not a pre-requisite (although you will become unstuck in due course if you don't gain skills). Like the LERC manager, you also need to be capable of motivating people, but more importantly you must understand how important it is to give structured feedback to your contributors: schemes that act as a black hole rapidly lose impetus (and there are good many such schemes). As a scheme organiser you must also be confident enough to challenge incoming records, no matter who from, so you MUST develop the necessary taxonomic, ecological and biogeographic skills. If you run a national scheme it also helps to have a reasonable knowledge of the landscape and ecology of the whole of the British Isles (but that can come with time).
What are you intending to produce with the data?
Here I think the biggest divergence probably obtains between the LERC and the Recording Scheme.
Unless the LERC holds data and is seen as a suitable source of information it will not get the income to survive - so financial survival is critical. You will not necessarily be judged on data quality - to many clients the important thing is enough information to satisfy the local planners that you have adequately investigated the environmental parameters of your proposal. For the NBN, an ability to list the vast numbers of records at a broad taxonomic scale is probably pretty important to attract ongoing statutory agency funding. In other words, nobody is starting from the question of data quality - it is headlines that grab the politicians and it is the politicians that control the purse-strings. And, as the vast majority of politicians have no scientific knowledge whatsoever and could not interpret a graph if it hit them in the face, big means best!
For the Recording Scheme, the most obvious and stinging criticism is that the dataset contains anomalies. The second most damaging problem is a failure to get data organised and maps and other outputs transmitted to your supporters (in other words they have a different set of supporters). If the supporters lose faith in the scheme then they will not engage and the scheme will decline. If it is vibrant and producing lots of outputs and creating a community around the interest group, more records will be generated and reliable recorders will be recruited. So, a vibrant recording scheme has got to be there to create a community spirit and to provide motivational feedback.
How many LERC and NBN staff run recording schemes or contribute records out of office hours?
I doubt this one can be quantified. I do know that at least some are occasional contributors to schemes. But in a parallel situation, I was always amazed at the low level of interest in biological recording amongst colleagues at Natural England. True, there were plenty of twitchers but real recorders were at a premium. Precious few former colleagues sit amongst the major or even minor contributors to the HRS (except former CSD and EFU staff who make up a significant part of the key major recorders). It was always a disappointment to me to hear that people felt that they did not want to take their job home with them but were happy to sit in meetings talking about what the recording schemes could be tasked with.
And the moral of the story?
Unless we get away from the volume rather than quality argument, there will be increasing question marks over the reliability of NBN data. That in turn means that Recording Schemes will be called upon for ever more data validation - which is already reaching unviable proportions. So, as a starting point perhaps LERCs and the NBN need to be developing lists of species that they can use as reality checks for datasets. If there are obvious dodgy records then the whole dataset should be disallowed until it has been validated - and if there is nobody available to validate it then simply mark the data as unvalidated and don't have them appear on the maps.Unless some attention is paid to data quality, users of the NBN will start to become wary of its content. If the data are unreliable, then so too are the outputs of the science that is based on those data. In the past, the HRS has flagged dodgy records with LERCs. Some have taken action, but others have ignored our advice - so we find it best not to access those datasets because we have to go through the same process time and again. We no longer bother to provide that feedback except where we know LERC managers will act.
Tuesday 5 December 2017
Whose data is it?
The Hoverfly Recording Scheme gets fairly regular requests for access to the dataset, mainly from University 'pollinator' groups. In general, we are happy to oblige and as a result the HRS data get used in all manner of ways. This is absolutely right - when we engage with recorders via Facebook we make it clear that the data are used in this way, so I hope nobody is in any doubt that we are assembling a dataset for wider usage and for the 'common good'.
Nevertheless, there is a fine dividing line between making data available for research purposes and simply being seen as the source of data upon which to build research proposals. Today, I got a request for access to the data from a PhD student who remarked that his PhD proposal was highly dependent upon access to the HRS dataset. Nobody had talked to us previously, so this came like a bolt out of the blue! OK, we will make the data available - after all, we are simply the custodians of the data and NOT the owners. We must not be protective apart from making sure that the data are used wisely and in the common good.
The problem I start to have is that the job of running a recording scheme for a popular group has evolved into pretty much a full-time occupation. I do not dare have a day off between about the end of February and the end of November and can anticipate putting in between 6 and 10 hours daily during the summer months. I know other schemes find it difficult to keep up with the demands on their time, so I cannot be the only one that feels the change. If running a scheme has become this demanding, there is a need to ask what motivates the recording scheme organiser and what will either:
I've got nothing to lose - my career has hit the rocks and in eight months time I will be able to draw my pension so I simply have to survive until then! So, I will say what others might be more reticent to say!
The other thing that motivates me is that after all these years running the scheme (26 years now since 1991) we now have a long enough data run to start to do some nice analytical work and to publish some interesting papers. I WANT to do just that - after all, I was trained as a scientist, I have a scientist's mind and I want to do something meaningful with the data. BUT, I must remember that we are simply custodians of the data and NOT the owners.
I reckon we should be aiming to retire from the front-line of running the scheme around 2021 (30 years tenure) and I would like to think that by then we will have produced a decent run of papers; but to do so we must pull our fingers out (that means me!)
To then find that the academic World sees us as simply a data resource, builds PhD or other grant bids based on access to the data we compile, but does not bother to talk to us first is somewhat irksome to say the least. To then see papers emerging in which the data come from us but the credits go to the academics is deeply frustrating. It is of course 'Citizen Science' - that great unwashed with no scientific expertise providing the great scientists with the material to produce their latest papers.
I also become increasingly demoralised to encounter ever-increasing attacks on anybody who has the temerity to post a photograph of a preserved specimen or to talk about specimens as 'material'. Why should I have to spend part of my time defending the collection of data that is the only facility available to show mankind the folly of our actions? It is as if the worst part of mankind is that which lacks morality and is actually prepared to generate reliable and meaningful data. Far better to rant at Governments without reliable data and then rant because you've been shot down for lack of legally admissible information!
I think it is time that the agenda changed from 'how to we motivate recorders to produce more data' to 'how do we maintain and improve the morale of the people that keep the recording schemes going?'
Rant over - but hopefully it sparks a meaningful debate!
Nevertheless, there is a fine dividing line between making data available for research purposes and simply being seen as the source of data upon which to build research proposals. Today, I got a request for access to the data from a PhD student who remarked that his PhD proposal was highly dependent upon access to the HRS dataset. Nobody had talked to us previously, so this came like a bolt out of the blue! OK, we will make the data available - after all, we are simply the custodians of the data and NOT the owners. We must not be protective apart from making sure that the data are used wisely and in the common good.
The problem I start to have is that the job of running a recording scheme for a popular group has evolved into pretty much a full-time occupation. I do not dare have a day off between about the end of February and the end of November and can anticipate putting in between 6 and 10 hours daily during the summer months. I know other schemes find it difficult to keep up with the demands on their time, so I cannot be the only one that feels the change. If running a scheme has become this demanding, there is a need to ask what motivates the recording scheme organiser and what will either:
- motivate them to keep going; or
- de-motivate them and lead to a loss of scheme activity?
I've got nothing to lose - my career has hit the rocks and in eight months time I will be able to draw my pension so I simply have to survive until then! So, I will say what others might be more reticent to say!
So, what motivates me?
These days, my main motivation is to try to make sure that by the time I pop my cloggs there is somebody to take over from me, Stuart and everybody else. A huge investment of time and emotional capital has gone into building the HRS from a pretty shaky base into one of the biggest invertebrate datasets in the UK (and probably one of the biggest Diptera datasets in the World). That investment will be wasted if we have no successors.The other thing that motivates me is that after all these years running the scheme (26 years now since 1991) we now have a long enough data run to start to do some nice analytical work and to publish some interesting papers. I WANT to do just that - after all, I was trained as a scientist, I have a scientist's mind and I want to do something meaningful with the data. BUT, I must remember that we are simply custodians of the data and NOT the owners.
I reckon we should be aiming to retire from the front-line of running the scheme around 2021 (30 years tenure) and I would like to think that by then we will have produced a decent run of papers; but to do so we must pull our fingers out (that means me!)
And what de-motivates me?
I have to say that I have become increasingly frustrated to get the impression that recording schemes are looked upon by all and sundry as a source of free data. That starts with the biodiversity industry that is always looking for new ways to increase the volume of biodiversity data without stopping to think about who will compile it, verify it, generate the enthusiasm amongst recorders, validate records and extract records. In practice, it has meant that an awful lot of schemes have turned from a private passion into an Albatross - you cannot drop it without there being dire consequences for something that you have invested half your life in (well almost) but if you don't drop it you have to invest even more because the demands are increasing.To then find that the academic World sees us as simply a data resource, builds PhD or other grant bids based on access to the data we compile, but does not bother to talk to us first is somewhat irksome to say the least. To then see papers emerging in which the data come from us but the credits go to the academics is deeply frustrating. It is of course 'Citizen Science' - that great unwashed with no scientific expertise providing the great scientists with the material to produce their latest papers.
I also become increasingly demoralised to encounter ever-increasing attacks on anybody who has the temerity to post a photograph of a preserved specimen or to talk about specimens as 'material'. Why should I have to spend part of my time defending the collection of data that is the only facility available to show mankind the folly of our actions? It is as if the worst part of mankind is that which lacks morality and is actually prepared to generate reliable and meaningful data. Far better to rant at Governments without reliable data and then rant because you've been shot down for lack of legally admissible information!
And the moral of the story?
I think it is time that the agenda changed from 'how to we motivate recorders to produce more data' to 'how do we maintain and improve the morale of the people that keep the recording schemes going?'
Rant over - but hopefully it sparks a meaningful debate!
Sunday 3 December 2017
Starting to retain specimens - a simple guide
Some photographic recorders start to want to know what they are missing out on - there are frequently species that cannot be done from photographs and it is frustrating not to know what they are, especially if you are trying to generate a picture of what occurs in a garden or favoured wildlife site. What can be done?
The option several members of the UK Hoverflies Facebook group have adopted is to collect specimens and store them for the autumn when they send them to me for identification. I have written about this before, but it is always worth an update.
There are several ways of doing this:
The simplest is to pop the container with your fly in it in the freezer for 24 hours - very few summer insects will survive such a time in the freezer.
An alternative is to use ethyl acetate or nail varnish remover - couple of drops on a piece of tissue popped into the container (beware that Ethyl Acetate is a solvent of some plastics, especially polystyrene which is often used. So, if in doubt use a small glass tube.
A further alternative is to take the fresh early growth from cherry laurel and crush it up into small fragments before putting as a deep layer in a tube or bottle and covering it with a wad of tissue paper - tightly pressed down. This is the traditional entomologists' 'killing bottle' and makes use of the cyanide released from these young leaves. I use this system for bigger flies, sawflies and other Hymenoptera (and any other big insects that are otherwise difficult to kill quickly).
In an earlier post I showed how John Bridges does this using little plastic envelopes. It is a great system but he and I did hit a bit of a problem with mould, so I think that the alternative is to use a breathable envelope - I have previously shown how to make these too but here is the sequence again:
These envelopes should be left in an open ventillated place for perhaps 24 hours so as to aid drying. After that I would store in a box - probably best to be breathable rather than a sealed plastic box that can build up condensation and mould - I have had problems in the past with mould and now leave my specimens in a more open situation for several days before using a cardboard box to store them.
I once sent an envelope of fungus gnats to Peter Chandler in a poorly protected package - he later wrote to say that they had arrived in many tiny pieces but he had managed to construct a list from the genital capsules of some of them! That was an object lesson for me, so I now send my samples in plastic boxes - the sort you get from a takeaway Chinese meal.
The option several members of the UK Hoverflies Facebook group have adopted is to collect specimens and store them for the autumn when they send them to me for identification. I have written about this before, but it is always worth an update.
Killing specimens
There are several ways of doing this:
The simplest is to pop the container with your fly in it in the freezer for 24 hours - very few summer insects will survive such a time in the freezer.
An alternative is to use ethyl acetate or nail varnish remover - couple of drops on a piece of tissue popped into the container (beware that Ethyl Acetate is a solvent of some plastics, especially polystyrene which is often used. So, if in doubt use a small glass tube.
A further alternative is to take the fresh early growth from cherry laurel and crush it up into small fragments before putting as a deep layer in a tube or bottle and covering it with a wad of tissue paper - tightly pressed down. This is the traditional entomologists' 'killing bottle' and makes use of the cyanide released from these young leaves. I use this system for bigger flies, sawflies and other Hymenoptera (and any other big insects that are otherwise difficult to kill quickly).
Storing specimens
In an earlier post I showed how John Bridges does this using little plastic envelopes. It is a great system but he and I did hit a bit of a problem with mould, so I think that the alternative is to use a breathable envelope - I have previously shown how to make these too but here is the sequence again:
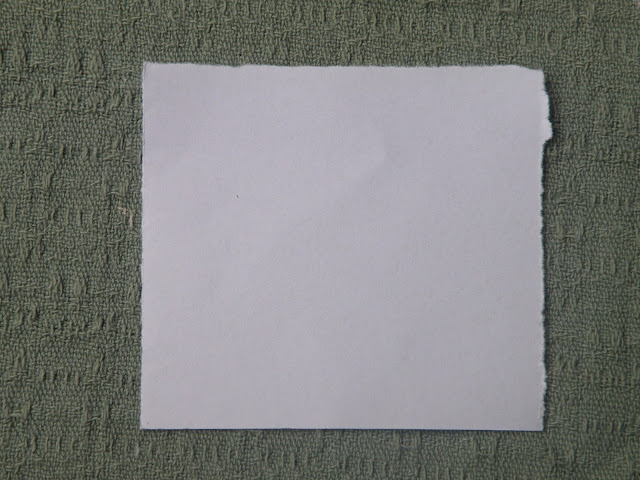 |
| Stage 1. cut a piece of paper about 7cm square |
 |
| Stage 2. Turn one side over to form a triangle |
 |
| Stage 3. Turn over one side to seal the edge - it is often a good idea to stick this down with a slip of masking tape. |
 |
| Stage 6. turn over the open end and again seal with masking tape. |
Postage
I once sent an envelope of fungus gnats to Peter Chandler in a poorly protected package - he later wrote to say that they had arrived in many tiny pieces but he had managed to construct a list from the genital capsules of some of them! That was an object lesson for me, so I now send my samples in plastic boxes - the sort you get from a takeaway Chinese meal.
What happens next?
When specimens reach me they go into a box of samples awaiting identification - which I do intermittently; which reminds me .......! I have several to do and had better get on with them today!And the benefits?
Eventually, you will get a spreadsheet back from me detailing what you have caught. This spreadsheet will also go on the HRS database so there is a more detailed account of species in your area. This in turn helps both distribution mapping and our knowledge of species' abundance and phenology. It also helps to improve the interpretations of distribution using predictive models that use occurrence data to predict distribution and to assess trends (e.g. Frescalo or Maxent).Saturday 2 December 2017
The perennial challenge - 'taking specimens is immoral'
From time-to-time somebody posts a photograph of a preserved specimen on one of the Facebook forums. These posts are often accompanied by calls for help with identification or are used for educational purposes and are often very useful for developing the skills of the more enlightened participants; many of whom use a range of guidebooks that have been illustrated using photographs or paintings of preserved specimens. Where would we be without those preserved specimens?
Well, in the case of hoverflies there would not have been Steve Falk's magnificent colour plates in the monograph on British hoverflies (now sadly rather out of date). Nor would we have the lavishly illustrated WILDGuide (Britain's Hoverflies). Stuart and I spent a good two years carefully photographing the critical features. We are now embarking on a new guide to British Flies (assuming a publication agreement) and will have a massive job provide the illustrations - many of which will have to be of recently killed specimens because they rapidly lose their critical colours and become wrinkled, thus obscuring important features. So, bottom line - no specimens = no guide book!
BUT, I think we ought to stop and think a bit further. Invertebrates are already the Cinderella of conservation - very few people take them seriously because the level of information available is so limited. It is as easy as anything for a developer to make a noise about a 5mm long wasp and howl that it is madness to stop a development on account of this animal - after all, fewer than 20 people in the country can either find or identify it! QED there is no conservation case!
Sadly. the vast majority of invertebrates are simply not identifiable from photographs. So they will always be the Cinderella at the party. BUT, if we don't have any data at all because we have vilified the people who can identify them and take the trouble to do so and to help others do so, we will have no defence at all for the vast majority of our fauna. I can hear the QC asking the quavering Natural England Officer 'and when was the last record of this species? Moreover, when was there anybody capable of identifying it?' - Believe me, that is how it works - these sorts of processes are highly intimidating. So to use that great quote from Sir Geoffrey Howe in his resignation speech (from the Thatcher Government) 'It's rather like sending our opening batsmen to the crease only for them to find that before the first ball is bowled, their bats have been broken by the team captain.' Every well-intentioned opponent of retaining specimens is like that team captain!
We all drive cars (well nearly all) and therefore we all contribute to the death of countless invertebrates - so much so that I suspect that road casualties are at least a factor in invertebrate decline. Likewise, we all rely on the supermarket for food and massive road miles that accompany it; likewise for our mobile phone, colour TV or iPad - everything we consume is accompanied by the death of countless invertebrates that generates nothing useful for science and nobody bothers to even acknowledge (see my post on Roadkill where I actually did a count).
It therefore seems to me that it is time to look long and hard at invertebrate conservation - is it the technical specialist who is having a significant impact? Is it the family of blue tits in your nest box? Maybe if we got rid of all the blue tits and entomologists the invertebrate world would be safe? Of course it would not! Reducing roadkill might go some way but still it would not. What would make a difference is a massive lifestyle change by all of mankind, but that will never happen, because it is OK to go twitching but morally unjustified to have a technical specialism that generates valuable scientific data!
Well, in the case of hoverflies there would not have been Steve Falk's magnificent colour plates in the monograph on British hoverflies (now sadly rather out of date). Nor would we have the lavishly illustrated WILDGuide (Britain's Hoverflies). Stuart and I spent a good two years carefully photographing the critical features. We are now embarking on a new guide to British Flies (assuming a publication agreement) and will have a massive job provide the illustrations - many of which will have to be of recently killed specimens because they rapidly lose their critical colours and become wrinkled, thus obscuring important features. So, bottom line - no specimens = no guide book!
BUT, I think we ought to stop and think a bit further. Invertebrates are already the Cinderella of conservation - very few people take them seriously because the level of information available is so limited. It is as easy as anything for a developer to make a noise about a 5mm long wasp and howl that it is madness to stop a development on account of this animal - after all, fewer than 20 people in the country can either find or identify it! QED there is no conservation case!
Sadly. the vast majority of invertebrates are simply not identifiable from photographs. So they will always be the Cinderella at the party. BUT, if we don't have any data at all because we have vilified the people who can identify them and take the trouble to do so and to help others do so, we will have no defence at all for the vast majority of our fauna. I can hear the QC asking the quavering Natural England Officer 'and when was the last record of this species? Moreover, when was there anybody capable of identifying it?' - Believe me, that is how it works - these sorts of processes are highly intimidating. So to use that great quote from Sir Geoffrey Howe in his resignation speech (from the Thatcher Government) 'It's rather like sending our opening batsmen to the crease only for them to find that before the first ball is bowled, their bats have been broken by the team captain.' Every well-intentioned opponent of retaining specimens is like that team captain!
Pause for reflection
I don't think I ever recall seeing anybody getting hot under the collar because Fred or Joe has assembled the longest list of twitches for 2017/16/15 .... Quite the opposite - they are the heroes of the conservation movement - great because they are so fanatical about wildlife. BUT, are they? Where is their wildlife legacy - I cannot say I will be rushing to the 'Museum of Tick Books' to catch a Glimpse of Bill Oddie's books or whomever else. What do they add to the sum of scientific knowledge - NOWT - they often are lists of places that they and several hundred or thousands of other people have used the earth's precious resources in achieving what? A list! And the cost in CO2 emissions?We all drive cars (well nearly all) and therefore we all contribute to the death of countless invertebrates - so much so that I suspect that road casualties are at least a factor in invertebrate decline. Likewise, we all rely on the supermarket for food and massive road miles that accompany it; likewise for our mobile phone, colour TV or iPad - everything we consume is accompanied by the death of countless invertebrates that generates nothing useful for science and nobody bothers to even acknowledge (see my post on Roadkill where I actually did a count).
It therefore seems to me that it is time to look long and hard at invertebrate conservation - is it the technical specialist who is having a significant impact? Is it the family of blue tits in your nest box? Maybe if we got rid of all the blue tits and entomologists the invertebrate world would be safe? Of course it would not! Reducing roadkill might go some way but still it would not. What would make a difference is a massive lifestyle change by all of mankind, but that will never happen, because it is OK to go twitching but morally unjustified to have a technical specialism that generates valuable scientific data!
Subscribe to:
Posts (Atom)


